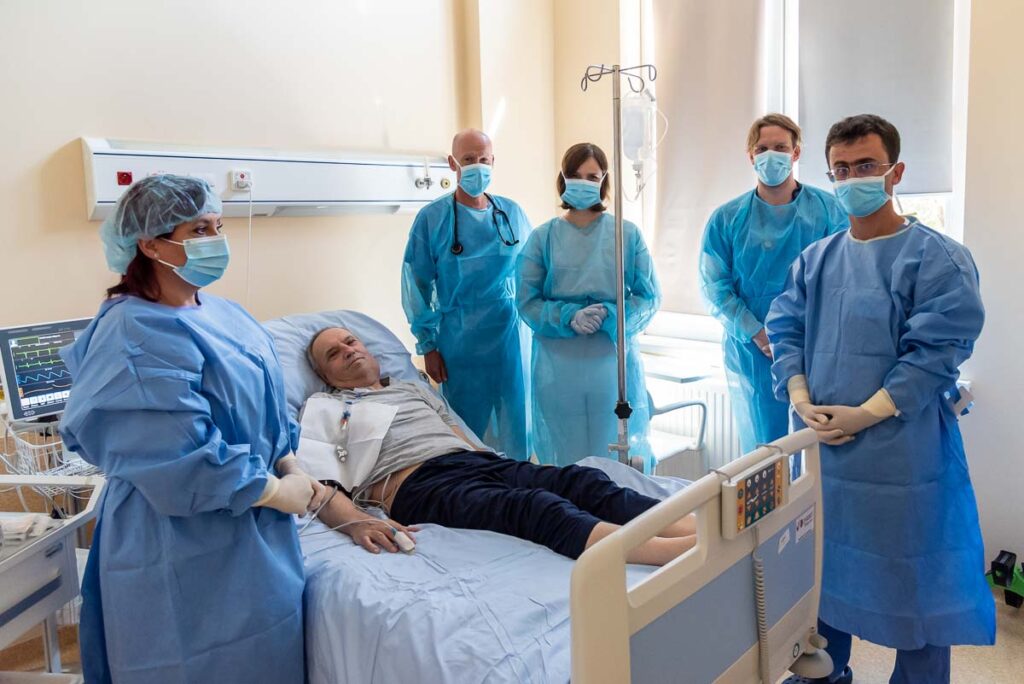
In August 2025, the Wroclaw Medical University made history by administering the first academic CAR-T cells in Poland to a patient with aggressive lymphoma. The breakthrough procedure was carried out at the Department of Hematology, Cellular Therapy and Internal Medicine, marking a milestone not only for the University but also for the entire Polish healthcare system. For the first time, a CAR-T therapy developed and manufactured in an academic center was successfully delivered to a patient.
This achievement was made possible thanks to funding from the Medical Research Agency (Agencja Badań Medycznych) and private donations supporting the clinic’s scientific efforts.
What is CAR-T therapy?
CAR-T therapy uses the patient’s own lymphocytes, which—after genetic modification—gain the ability to recognize and destroy cancer cells. In the case of lymphoma, the modified cells target the CD19 antigen, effectively eliminating malignant cells that carry it.
Key advantages of academic CAR-T
Unlike commercial CAR-T products offered by pharmaceutical companies, the academic version offers several important benefits:
- Lower cost – at least three times cheaper to produce.
- Shorter manufacturing time – the process from cell collection to infusion can be completed in just three weeks, twice as fast as commercial products.
- Broader access for patients – including those over 70 years of age and patients with less common lymphoma subtypes who are not eligible for reimbursement under commercial programs.
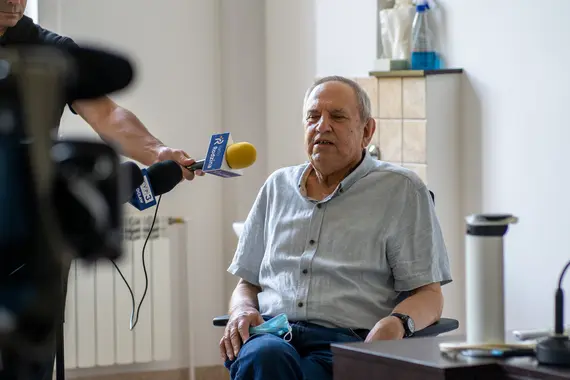
The first patient treated in Wrocław was a 76-year-old man with relapsed aggressive lymphoma, who would not have qualified for commercial CAR-T therapy.
Five years of preparation
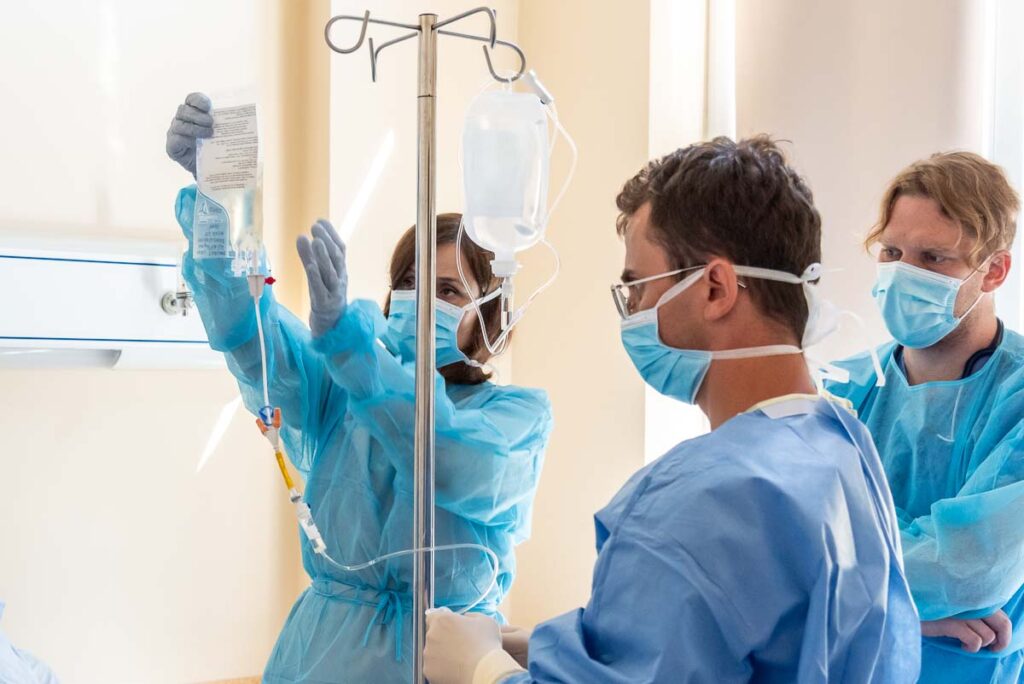
The project was supported by a nearly 15 million PLN grant from the Medical Research Agency, as well as 1.1 million PLN in private donations from patient families. These early contributions enabled the purchase of essential equipment and reagents, laying the groundwork for research and development.
Collaboration with the Regional Blood Donation and Hemotherapy Center (RCKiK) in Poznań proved crucial for the project. CAR-T cells are produced there in specialized facilities and then transported back to Wrocław for administration. The production process takes 14 days, and the entire procedure from collection to infusion lasts about 21 days.
Looking ahead
The clinical study in Wrocław focuses on patients with aggressive B-cell lymphomas, the most common type in Poland, affecting around 1,000–1,500 people annually. The trial will enroll 9–18 patients nationwide over the next 18 months.
“Our goal is not only to broaden access to CAR-T therapy but also to build a stable platform for further university-led research and innovation,” emphasized Prof. Tomasz Wróbel, Head of the Department
In the future, the platform may be extended to other indications, including acute lymphoblastic leukemia in children, multiple myeloma, Hodgkin lymphoma, acute myeloid leukemia, and even autoimmune diseases or solid tumors such as gliomas.
The project highlights the role of academic centers in expanding therapeutic options beyond the scope of commercial programs, fostering independence from foreign technologies, and creating opportunities for new patents and biotechnological development in Poland.
Photo credit: Tomasz Walów, Tomasz Modrzejewski / Wroclaw Medical University